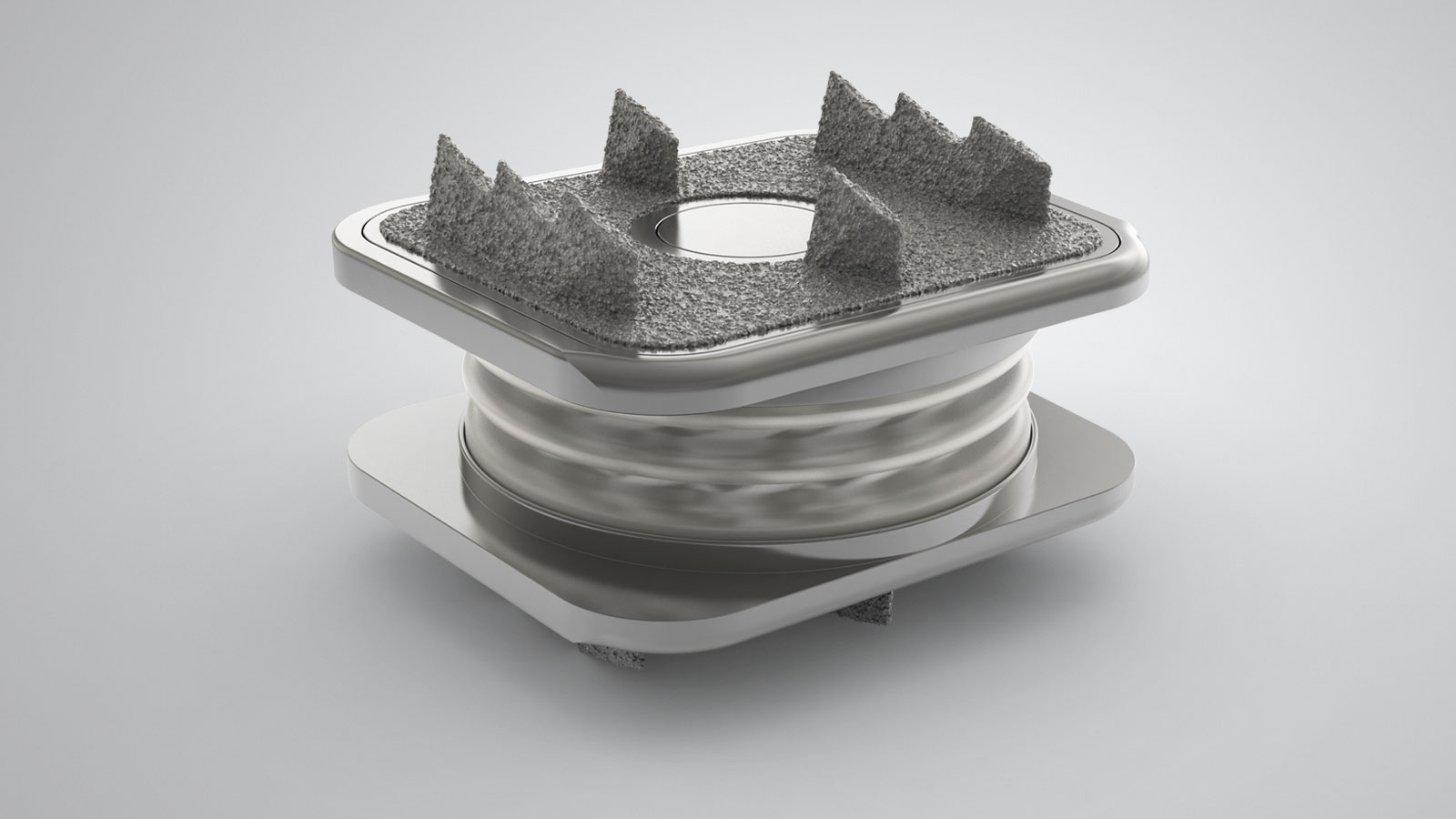
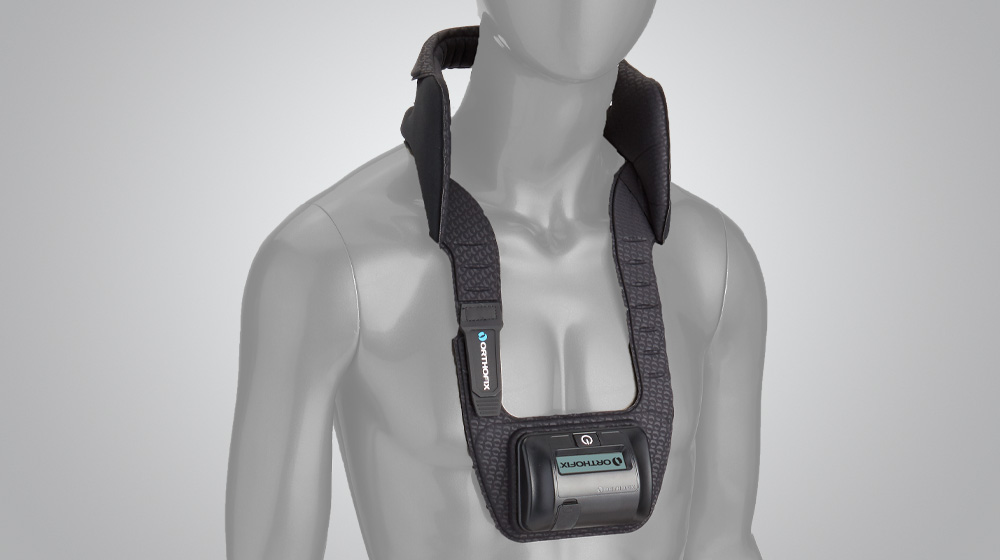
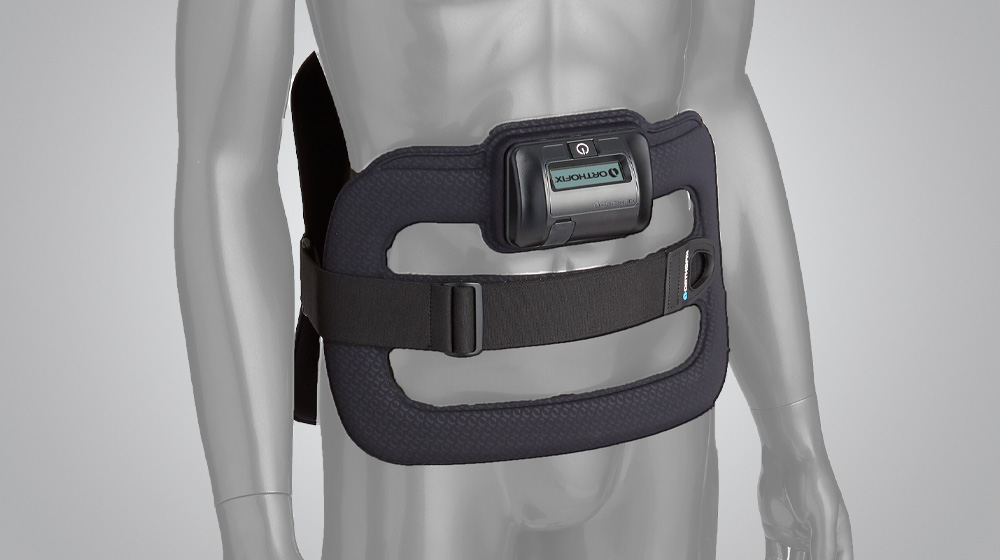
At Orthofix, we are committed to developing new products in all areas of our business, and we do this through Investigational Device Exemption studies (IDEs), as well as post-approval and post-market studies. IDE studies are used to bring new products or existing products with new indications to market. Post-market studies allow us to examine the effectiveness of our products in a real-world setting. In a post-market study, physicians follow their patients for a period time so that the effectiveness of a device or product can be further evaluated by Orthofix.